Unlocking Blood-Brain Barrier Boosts Immunotherapy Efficacy, Lowers ARIA
Quick Links
Current amyloid immunotherapies poorly enter the brain, with only one in a thousand antibodies getting through. For several years, researchers have been exploring alternate delivery methods that could allow for lower dosing and higher potency. Some fruits of that labor were on display at last month’s Clinical Trials on Alzheimer’s Disease conference, held October 24 to 27 in Boston and online. Roche scientists debuted the first efficacy and safety data for their new antibody trontinemab, which links gantenerumab to a “brain shuttle” that ferries it across the blood-brain barrier. In a small study of 44 people, trontinemab exceeded Roche’s expectations, completely clearing plaque from three-quarters of participants within six months.
- Trontinemab rapidly clears plaque with little ARIA.
- Potentially, this is because its direct brain delivery method sidesteps vascular amyloid.
- Breaching the blood-brain barrier via ultrasound also boosts antibody efficacy.
Another talk presented a different way to pierce the barrier—using ultrasound to stretch vessel walls in local areas, making them leaky enough for aducanumab to pass. This strategy cleared half of deposits in those regions within six months.
Importantly, preliminary results suggest these methods may cause less ARIA than conventional antibody delivery. Why? Scientists showed that circulating antibodies end up in cerebrospinal fluid long before they reach the brain’s parenchyma. CSF communicates with perivascular spaces around large arteries, where cerebral amyloid angiopathy abounds. Thus, vascular amyloid may be the first form that conventional antibodies encounter. Delivering antibodies directly through the blood-brain barrier avoids this danger, because smaller vessels have less CAA. Other talks in Boston hammered home that ARIA represents an inflammatory reaction to CAA, an idea catching fire in the field (Aug 2023 conference news).
Per-Ola Freskgård of BioArctic called the brain shuttle data fantastic. He previously worked on the program at Roche, and is now developing a similar shuttle at BioArctic. “It’s a game-changer, not only in Alzheimer’s disease but for the use of biologics for brain disorders in general,” he told Alzforum. “I think the whole field will move into this space of active transport of antibodies.”
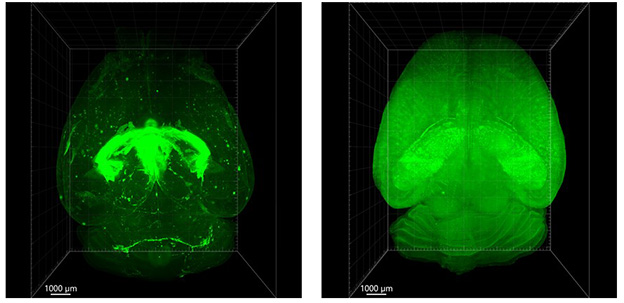
Conventional versus Shuttle. In mouse brain, conventional antibodies (green, left) linger in the choroid plexus and ventricles five days after infusion. A brain shuttle (right) delivers them evenly throughout brain parenchyma. [Courtesy of Roche.]
Meet the New Kid—Trontinemab Struts Its Stuff
Roche’s brain shuttle is a Fab antibody fragment that binds the transferrin receptor. After binding this receptor on vascular endothelial cells, the shuttle and its cargo are internalized, passing through the blood-brain barrier via active transport. Previously, Roche had reported data from a Phase 1 single-ascending-dose study, which found eightfold higher brain uptake of trontinemab than gantenerumab (Mar 2021 conference news). Analyzing pharmacokinetic data, the scientists predicted that 3.6 mg/kg monthly intravenous trontinemab might lower plaque load by a quarter after six months (Dec 2021 conference news).
In Boston, Roche’s Luka Kulic reported interim data from a multiple-ascending-dose study. It turned out trontinemab handily beat that estimate. The Phase 1b/2a trial has so far enrolled 44 participants who have mild cognitive impairment or mild dementia due to AD. Participants were 70 years old on average, with an average MMSE of 20 and between 90 and 100 centiloids of plaque at baseline. To date, the trial has tested doses of 0.2, 0.6, and 1.8 mg/kg; each dose cohort consists of about 15 people, of whom three receive placebo. For comparison, the gantenerumab Graduate trials administered roughly 15 mg/kg monthly, eight times more than the highest dose here.
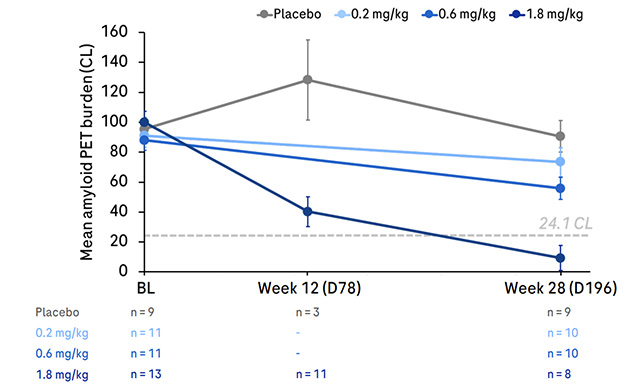
Going, Going, Gone. Six months of trontinemab lowered amyloid dose-dependently, with the highest dose of 1.8 mg/kg (dark blue) completely clearing plaque. [Courtesy of Roche.]
The results? After six months, people receiving 0.2 mg/kg had lost about 20 centiloids, those on 0.6, 31. At the 1.8 mg/kg dose, plaque clearance accelerated dramatically, with 62 centiloids having vanished by three months and 84 centiloids by six. At this dosage, three of four participants dropped below the amyloid positivity threshold of 24.1 centiloids (see image above). A fourth and final dose cohort getting 3.6 mg/kg is now fully enrolled; those data will be presented next year, Kulic added.
Based on these data, Freskgård estimated trontinemab’s efficacy might be 40- or 50-fold that of gantenerumab.
The treatment produced side effects, but none so far that are showstoppers. Most common were infusion-related reactions; they occurred in three of four participants on the 1.8 mg/kg dose. This typically happened at the first infusion, and symptoms were usually mild, consisting of fever and chills. The researchers now give acetaminophen to participants beforehand to ameliorate this reaction.
In addition, 19 percent of people on 1.8 mg/kg developed mild anemia. This could be a result of the brain shuttle construct interfering with iron uptake in red blood cells. It could also be a result of the frequent blood draws in this study, because the placebo group had mild anemia as well, Kulic noted. Because anemia was most common in people whose iron levels were low at baseline, these participants are now being pretreated with iron supplements to bring them up to normal before starting trontinemab. Anemia went away after trontinemab treatment stopped.
Trontinemab also induced anti-drug antibodies. Curiously, ADAs were most pronounced at lower doses, curtailing trontinemab exposure by 70 percent at 0.2 mg/kg, 60 percent at 0.6, and 25 percent at 1.8. Kulic told Alzforum that this phenomenon of bigger ADA effects at lower doses has been seen with other drugs, for example in oncology, but is not fully understood. ADAs were not associated with ill effects in this study, he added.
Now for the big question—what about ARIA? Here the news was good. At the lower two doses, there were no incidents. At 1.8 mg/kg, one person developed ARIA-E, and one ARIA-H, for an incident rate of 7 percent each. The ARIA-E case was mild on MRI, affected the person’s ability to pay attention, and went away after a month. The ARIA-H was asymptomatic and consisted of two occurrences of the leakage called meningeal siderosis.
If the findings hold up in larger cohorts, this would be the lowest rate of ARIA-E yet seen with amyloid immunotherapy. Other antibody programs have reported rates ranging from 12 to 33 percent. Gantenerumab, which forms the backbone of trontinemab, triggered ARIA-E in 22 percent of people in its Phase 3 trial.
Pinning the Blame on Vascular Amyloid
Why does trontinemab cause less ARIA? Possibly, because it enters the brain differently than do gantenerumab and other standard anti-Aβ antibodies. At a task force meeting before CTAD, Kulic presented mouse data pinpointing where in the brain fluorescently labeled antibodies accumulated five days after dosing. A conventional IgG antibody directed against a neuronal target was found mostly in the choroid plexus, ventricles, and subarachnoid space—in other words, in CSF. That same antibody conjugated to the brain shuttle, by contrast, had broadly diffused throughout the brain, including deep brain structures (see image at top of story).
Freskgård believes that conventional antibodies enter the brain through the choroid plexus, because this tissue’s job is to filter blood. Though a blood-CSF barrier exists, consisting of tight junctions between the ependymal cells that line the choroid plexus, he thinks this hurdle is easier for large molecules to cross than is the blood-brain barrier. Once in CSF, antibodies presumably circulate through ventricles and the subarachnoid space, eventually entering the brain through the perivascular spaces that surround large penetrating arteries. These arteries tend to be laden with the most CAA. Because of this, conventional antibodies likely encounter vascular amyloid long before they see any parenchymal plaques, Freskgård speculated.
At CTAD, Cynthia Lemere of Brigham and Women’s Hospital, Boston, showed unpublished data that supports this idea. When her team injected amyloidosis mice with a standard anti-amyloid antibody once weekly for three weeks, then immunostained for it, they found nearly all the signal around blood vessels in the brain. When they treated for 13 weeks, they found immunoreactivity in plaques as well as blood vessels. The data suggest that antibodies associate with vascular amyloid first, and do so for quite some time, before they reach the parenchyma. That may explain why ARIA develops early in treatment, and why it associates with CAA, Lemere suggested. “Binding of antibodies to CAA is critical for ARIA,” she said in Boston.
Trontinemab, by contrast, is taken up by transferrin receptors in capillaries that pervade the brain everywhere, giving it broad, rapid access to parenchyma. Capillaries carry much less vascular amyloid than do leptomeningeal arteries, potentially limiting the antibody’s exposure to such deposits. This may be why trontinemab causes less ARIA than does gantenerumab, Kulic noted. He added that Roche is exploring other possibilities as well, such as a lower systemic dose or a different pharmacokinetic profile playing a role.
Other talks at CTAD reinforced these ideas. Lars Lannfelt of Uppsala University, Sweden, compared the binding characteristics of lecanemab and donanemab. Both antibodies bound synthetic fibrillar amyloid spiked with pyroglutamated Aβ with similar affinity and, as expected, donanemab did not bind when there was no pyroGluAβ. When it came to CAA deposits, however, donanemab bound with about twice the affinity of lecanemab, as seen by three different assays: immunoprecipitation, surface plasmon resonance, and a type of electrochemiluminescent ELISA. The relative CAA binding strength of each antibody correlates with the amount of ARIA-E it triggers—12 percent for lecanemab, 24 for donanemab—again suggesting a link, Lannfelt said.
Meanwhile, Gioacchino Curiale of Biogen reinforced the evidence that ARIA is an early phenomenon. He presented data from Embark, aducanumab’s ongoing OLE study. Among 1,578 people with MRI safety data, 586 had previously received at least one dose of 10 mg/kg aducanumab in a trial, 784 had never reached the maximum dose, and 208 had previously been on placebo. Curiale found dramatic differences in ARIA rates between these groups, especially comparing participants with full dosing and those with none. ARIA-E rates were four times higher in the treatment-naïve group, 40 percent versus 11, and ARIA-H rates were twice as high, 38 percent versus 18.
ARIA was more severe in those new to treatment, with 22 versus 12 percent of ARIA-E cases symptomatic, and 7 versus 0 percent severe on MRI scans. Those new to treatment were also more likely to have ARIA recur during the three-year study, at 17 versus 3 percent. Participants who had previous low aducanumab exposure fell in between these groups on all measures.
Within the subgroup of those previously treated with aducanumab, however, people who had ARIA-E before were three times as likely to develop it in the OLE as were those who’d never had it, suggesting some underlying vulnerability.
Dennis Selkoe of BWH called these data important, and wondered if the lower ARIA rates in those with previous exposure were due to their starting the OLE with lower baseline amyloid. Curiale said this was likely. This would fit with a model in which removal of vascular amyloid during initial treatment made these participants less susceptible to ARIA.

ARIA Risk Factors. Machine learning identified six variables that affected a person’s odds of ARIA with immunotherapy, five risk and one protective. [Courtesy of Steven Greenberg.]
Further tying ARIA to CAA, Steven Greenberg of Massachusetts General Hospital, Boston, reported that factors that contribute to ARIA risk are all associated with vascular amyloid deposits. Using data from the donanemab Phase 2 and 3 studies, he applied machine learning to parse the contribution of 42 different variables, finding six that correlated with ARIA-E. No surprise, APOE genotype was the strongest risk factor, with two copies of the E4 allele tripling the odds of ARIA-E. Microhemorrhages were the next strongest; having even one at baseline boosted the odds by 40 percent, while two to four microhemorrhages more than doubled risk. Most trials allowed people with up to four baseline microhemorrhages to enroll. Likewise, the presence of one instance of superficial siderosis at baseline doubled the risk of ARIA-E. CAA is more common in E4 carriers, and frequently causes small brain bleeds.
The fourth risk factor was a surprise, however. Having a mean arterial pressure greater than the population norm of 93 raised the risk of ARIA-E by 40 percent, Greenberg reported, noting this is a new finding. Conversely, the use of antihypertensive medication dropped the risk by 40 percent, suggesting hypertension is a modifiable risk factor. Finally, high baseline amyloid of more than 108 centiloids also nudged up risk by 30 percent.
Microbubbles Make Plaque Go ‘Poof’?
Another talk at CTAD proposed a different way to foil the blood-brain barrier. Ali Rezai of West Virginia University, Morgantown, presented a pilot study of whether ultrasound could get more antibody in. Previously, low-intensity ultrasound directed to spots in the brain had temporarily opened the barrier in AD patients without obvious ill effects. The method uses microbubbles injected into the bloodstream; when an ultrasound pulse hits a vessel, the bubbles inside expand, pushing endothelial cells apart. These openings reseal within 24 to 48 hours (Aug 2018 news).
Rezai tested whether this method would boost uptake of aducanumab, the only approved amyloid immunotherapy at the time. For six months, three AD patients whose plaque load reached 200 centiloids received a monthly aducanumab infusion and lay for two hours of ultrasound in an MRI scanner. Ultrasound beams were targeted to brain regions of 40 mL in size; the same region of the opposing hemisphere served as control. Aducanumab was titrated up from 1 to 6 mg/kg during the study, below the effective dose of 10 mg/kg established in Phase 3 trials.
The procedure mopped up plaque quite well. Each participant cleared about half their baseline amyloid in the targeted regions within six months. No ARIA was seen, but APOE4 carriers were excluded. The three people will be followed for five years for long-term safety and efficacy.
Rezai is now enrolling a larger study that will infuse lecanemab and direct ultrasound to larger brain regions. One way or another, scientists have their eyes on conquering the blood-brain barrier.—Madolyn Bowman Rogers
References
Therapeutics Citations
News Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
The University of Queensland
Using low-intensity ultrasound to deliver anti-Aβ antibodies to the brain is indeed a promising strategy to increase antibody levels in the brain (and reduce ARIA).
We have shown previously that ultrasound achieves a fivefold increased brain uptake of an aducanumab analog in APP23 mice, along with improved cognitive outcomes (Leinenga et al., 2021). A similar finding has been obtained by Sun and colleagues, targeting pyroglutamate-3 Aβ (Sun et al., 2021). In the mice, Aβ has been reported by us and others to be cleared by microglia (Jordão et al., 2013; Leinenga et al., 2023).
It would be interesting to know how Aβ is being cleared in the human study participants to whom aducanumab was delivered with ultrasound. Is it via microglia or by facilitating glymphatic drainage or is it via yet another mechanism?
References:
Leinenga G, Koh WK, Götz J. A comparative study of the effects of Aducanumab and scanning ultrasound on amyloid plaques and behavior in the APP23 mouse model of Alzheimer disease. Alzheimers Res Ther. 2021 Apr 9;13(1):76. PubMed.
Sun T, Shi Q, Zhang Y, Power C, Hoesch C, Antonelli S, Schroeder MK, Caldarone BJ, Taudte N, Schenk M, Hettmann T, Schilling S, McDannold NJ, Lemere CA. Focused ultrasound with anti-pGlu3 Aβ enhances efficacy in Alzheimer's disease-like mice via recruitment of peripheral immune cells. J Control Release. 2021 Aug 10;336:443-456. Epub 2021 Jun 26 PubMed.
Jordão JF, Thévenot E, Markham-Coultes K, Scarcelli T, Weng YQ, Xhima K, O'Reilly M, Huang Y, McLaurin J, Hynynen K, Aubert I. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol. 2013 Oct;248:16-29. Epub 2013 May 21 PubMed.
Leinenga G, Bodea LG, Schröder J, Sun G, Zhou Y, Song J, Grubman A, Polo JM, Götz J. Transcriptional signature in microglia isolated from an Alzheimer's disease mouse model treated with scanning ultrasound. Bioeng Transl Med. 2023 Jan;8(1):e10329. Epub 2022 May 14 PubMed.
Make a Comment
To make a comment you must login or register.