Over the Span of AD, Roles of Astrocytes and Microglia Change
Quick Links
As neuronal supporters and immune surveyors, astrocytes and microglia are no mere bystanders to the neuronal mayhem that unfolds in Alzheimer’s disease. At the AD/PD meeting, held March 5-9 in Lisbon, scientists presented new twists on the relative contributions of the two cell types to various aspects of AD, including plaque and tangle accumulation, synaptic deterioration, brain atrophy, and cognitive decline. The upshot? Reactive astrocytes spell trouble for neurons and their synapses, and this holds true even among people who have no Aβ plaques. Microglia, on the other hand, transform from neuroprotectors to slayers only once plaques inundate the brain. In mouse models, where researchers could deplete and restore the cells, microglia polarize into several transcriptional states over the course of amyloidosis. These evolving states are paralleled by functional transitions, in that the cells initially seed Aβ plaques, and then compact them later on.
- Astrocyte reactivity links amyloid and tangle pathology, and associates with synaptic degeneration and cognitive decline.
- After plaques emerge, microglial activation correlates with synaptic loss and neurodegeneration.
- In mice, microglia seed plaques early in disease, and then compact them later.
Several presentations that attempted to disentangle the role of astrocytes versus microglia came from collaborators and scientists in the lab of Tharick Pascoal at the University of Pittsburgh. The researchers looked for connections between fluid and imaging biomarkers of Aβ and tau pathology, glial activity, and synaptic loss among several human AD cohorts.
Bruna Bellaver, an assistant professor in Pascoal’s lab, studies how astrocyte reactivity contributes to AD pathophysiology. Previously, she reported that among cognitively healthy people who have amyloid plaques, tangle pathology only showed up on PET scans among those whose astrocytes were fired up, as gauged by elevated plasma GFAP (Jun 2023 news). As she described in Lisbon, Bellaver has extended this line of work to decipher how astrocyte reactivity mediates correlations among plaques, plasma p-tau217, ApoE, and cognitive impairment across the AD spectrum
Bellaver’s newest analysis relied on data from more than 2,000 participants in four cohorts in the U.S., Canada, South Korea, and Chile. About half were cognitively healthy, and half had been diagnosed with mild cognitive impairment or AD. First, Bellaver investigated how astrocyte reactivity influenced the relationship between amyloid status, as gauged by PET or plasma Aβ42/40 levels, and plasma p-tau217. She found that across the AD continuum, plasma p-tau217 increased as a function of plaque burden, but only if astrocytes were reactive, as gauged by elevated plasma GFAP. In those with calm astrocytes, plaques did not rouse plasma p-tau217. This mediating effect was strongest among people who were cognitively impaired. These glia also mediated ApoE effects, such that ApoE4 potentiated the plaque-driven plasma p-tau217 only in people with reactive astrocytes.
Finally, Bellaver reported that astrocyte reactivity determined the heft of the cognitive blow dealt by amyloid and tau pathologies. While MMSE scores were unaffected by amyloid or p-tau217 alone, they dropped if reactive astrocytes accompanied either marker (image below). People with all three—Aβ plaques, high p-tau217, and reactive astrocytes—fared worst.

The Astrocyte Factor. Astrocyte reactivity (Ast+) potentiates the effect of amyloid (A+) and/or p-tau217 (Ptau+) on cognition (MMSE). [Courtesy of Bruna Bellaver, University of Pittsburgh.]
Bellaver speculates that astrocytes change when they sense amyloid buildup in the brain. “They get reactive and progressively lose neuroprotective functions and/or gain novel neurotoxic properties, disrupting brain homeostasis,” she wrote to Alzforum. This reactivity might preclude their ability to contain tau pathology, she speculated.
In Pascoal’s lab, Francieli Rohden used biomarker data to explore a related question: How do astrocytes and microglia contribute to synaptic loss that worsens over the course of AD? Rohden examined relationships among CSF biomarkers measured in 105 participants in the TRIAD cohort, and 373 from ADNI. Volunteers in both cohorts were classified based on cognitive and amyloid status. Rohden used CSF GFAP to gauge astrocyte reactivity, soluble TREM2 as a proxy for microglial activation, and pre- and post-synaptic proteins GAP43 and neurogranin released into the CSF as markers of damaged synapses. At AD/PD, Rohden reported that astrocyte reactivity correlated with both markers of synaptic destruction, regardless of amyloid or cognitive status.
The story was different for microglial activation, which only associated with GAP43 and neurogranin among people who had amyloid. In these participants, sTREM2 rose in step with GAP43, regardless of cognitive status, and with neurogranin among those who were cognitively impaired. These associations hinted that microglial activation might spur deterioration of pre-synapses earlier in disease, eventually compromising post-synapses later.
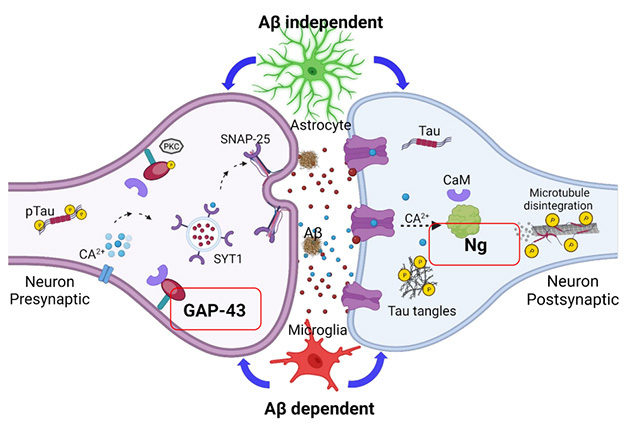
Showdown at the Synapse. Reactive astrocytes (top) and activated microglia (bottom) influence synaptic deterioration, as measured by the release of GAP43 from pre-synapses (left) and neurogranin from post-synapses (right). [Courtesy of Francieli Rohden, University of Pittsburgh.]
What explains ties between glial activity and synaptic damage? Rohden found that levels of CSF p-tau181 could fully predict the association between astrocyte reactivity and synaptic markers, regardless of Aβ or cognitive status. CSF p-tau181 also linked microglial activation with synaptic markers, but only among those with amyloid. Given that CSF p-tau181 is strongly linked to amyloid, how might it connect astrocytes to synaptic damage among those without a substantial plaque burden? The scientists speculated that Aβ oligomers might rile astrocytes, which then instigate the phosphorylation of tau. When p-tau congregates in pre-synapses, it gloms onto synaptic vesicles and might erode synaptic integrity.
One interpretation of Rohden’s findings is that once plaques are established in the brain, microglial activation becomes a liability. Shifty microglia were the focus of several presentations in Lisbon. Guilherme Povala, also of the Pascoal lab, used data from the TRIAD cohort to assess how activated microglia—this time, measured by TSPO-PET—related to brain atrophy throughout the AD continuum. TSPO is an outer mitochondrial membrane protein that rises in activated microglia, and can be measured with the tracer [11C]PBR28. For several regions of interest across the brain, Povala looked for associations between TSPO-PET and brain atrophy, which was measured with serial MRI scans over two years in 80 normal and 54 cognitively impaired volunteers.
What did he discover? Among those with normal cognition, the relationship between microglial activation and brain atrophy depended on amyloid status. In those without plaques, TSPO-PET correlated with higher regional volume, suggesting microglial activation was protective. In people with plaques, the relationship flipped, such that regions with higher TSPO-PET had more atrophy.
Among those with impaired cognition, all of whom had Aβ plaques, Povala uncovered a similar relationship, this time relative to tangle pathology. In people whose tangles were limited to early Braak stage regions, microglial activation correlated with more brain volume. The opposite was true when tau tangles had spread into later Braak regions, beyond the temporal lobe. For those people, riled microglia associated with brain shrinkage. Finally, Povala reported that regardless of clinical disease stage, microglial activation in the presence of amyloid and tangles predicted future cognitive decline.
Delphine Boche of the University of Southampton, U.K., noted that the findings seem consistent with a cascade in which Aβ triggers microglial activation, which then promotes tangle pathology and neurodegeneration. Povala agreed, adding that it appears microglia mount two distinct reactions, first to amyloid and then to tau. Interestingly, although they used two different markers for microglial activation, Povala and Rohden’s findings fit together, as both cast microglia in the amyloid-ravaged brain as a neurodegenerative force. At last year’s AD/PD meeting, researchers had suggested that perhaps TSPO reflects a harmful form of microglial activation (May 2023 conference news).
To better understand the effects of elevated TSPO, Boche used immunohistochemistry to characterize cells in postmortem brain samples from 60 people who had died with varying degrees of plaque and tangle pathology. In Lisbon, she reported that in microglia, TSPO congregated around nuclei and sometimes appeared in cytoplasmic processes, consistent with its mitochondrial localization. Curiously, TSPO was also occasionally expressed by smooth muscle endothelial cells lining blood vessels, but was not found in astrocytes or perivascular macrophages. TSPO increased in the temporal lobe with increasing Braak stages, and was not found in the cerebellum, where amyloid, but not p-tau, accumulated. Co-staining with antibodies for other microglial markers revealed that TSPO+ microglia tended to express CD68 or macrophage scavenging receptor-A (MSR-A), markers of phagocytic and scavenging microglia, respectively. Boche believes that TSPO+ microglia may be responding to p-tau and/or to neurodegeneration in AD.
Mariko Taga of Columbia University in New York also scrutinized postmortem brain sections to hunt for disease-related microglial phenotypes. Specifically, she wanted to know how microglia with ramped-up expression of CD74—a cell surface receptor that stokes antigen presentation—were related to different AD traits. Previous transcriptomic studies identified CD74 among elevated genes in disease-associated microglial subsets (Dec 2020 news). Because microglia are a diverse lot and make up only a small fraction of cells in the brain, Taga and colleagues developed an automated technique to scan an entire brain section at high resolution, pick out all of the microglia based on their expression of Iba1, and then study other features of the cells (De Jager et al., 2024). In Lisbon, Taga detailed her findings, applying the technique to dorsolateral prefrontal cortex sections from 64 donors in the ROSMAP cohort, and 91 from Columbia University’s brain bank. The analysis illuminated nearly 500,000 microglia within samples from both cohorts. In addition to Iba1, the sections were immunostained for GFAP, p-tau, and CD74. S
First and foremost, Taga found no major differences in the density of microglia based on pathological diagnosis. Across all Iba1+ microglia, expression of CD74 and Iba1 were strongly correlated, suggesting CD74 rises in step with microglial activation. Taga zeroed in the microglia with extraordinarily high CD74, i.e., more than two standard deviations above the mean. These cells were found in all of the samples, and did not increase in proportion across Braak stages. This suggested that tau pathology did not spur expansion of the cells. Oddly, the proportion of CD74-hi microglia did correlate with clinical diagnosis of AD, and with the rate of cognitive decline. The findings hint that CD74-hi microglia somehow potentiate the cognitive blow inflicted by AD pathology.
Tracking Human Microglia … in Mice
Microglia are known to have an intimate, and tumultuous, relationship with Aβ plaques. Researchers have extensively probed this interaction in mouse models by depleting microglia. As Nóra Baligács of KU Leuven in Belgium pointed out in her talk in Lisbon, conclusions have conflicted, with some casting microglia as plaque builders, and others as plaque destroyers (for example, Zhao et al., 2017; Jul 2019 conference news; Sep 2019 news). The differing results have been chalked up to differences in mouse models and microglia depletion protocols. However, Baligács, a graduate student in Bart De Strooper’s lab, hypothesized that answer might lie in timing—microglial involvement in plaque deposition may change throughout the disease process.
To test this idea, she treated APP-NL-G-F knock-in mice, which start to develop Aβ plaques just prior to 2 months of age, with the CSF1R inhibitor PLX3397 at different ages. Microglia rely upon this receptor for their survival, and inhibiting it docked their numbers by 83 percent. When this microglial culling was done in 1-month-old mice, before plaques had formed, Baligács found fewer plaques, and an overall lower plaque burden, in 4-month-old mice than in untreated NL-G-F mice. This early depletion of microglia also substantially reduced the amount of insoluble Aβ peptides detected in brain extracts, and curbed the development of dystrophic neurites. In contrast, depleting microglia when the mice were 3 months old—and plaque-ridden—resulted in a higher overall plaque burden. This boost was driven by an increase in plaque size, rather than plaque numbers, Baligács reported. Depriving mice of microglia at this later stage also exacerbated the numbers of dystrophic neurites.
Baligács thinks the findings reflect microglia’s shifting role from plaque seeders to plaque compactors. In support of this idea, depriving mice of microglia from 1 to 7 months of age resulted in a combination of effects: Mice had fewer plaques, but they were larger than in their microglia-replete counterparts.
To validate their inhibitor findings in a genetic model of microglial depletion, the researchers turned to FIRE mice. These animals have a deletion in the enhancer for the CSF1R receptor gene, resulting in a lack of receptor expression and completely snuffing out microglial survival, while macrophages remain unscathed. These animals were bred on an APP-NL-G-F background, and were also rendered immunodeficient, to allow for transplantation of human microglia. At both 6 weeks and 3 months of age, these microglia-deficient FIRE mice had fewer plaques than NL-G-F mice, again casting microglia as plaque seeders. When Baligács transplanted human microglia into the brains of FIRE mice a few days after they were born, the cells repopulated the brain. What’s more, by 3 months of age, plaque numbers increased relative to mice without the xenotransplants, though not to levels in age-matched NL-G-F mice. The findings further support the idea that microglia, and in this case human ones, build plaques.
Baligács plans to use these chimeric models to study the contributions of microglia throughout amyloidosis, and to investigate how different AD risk genes in human microglia influence this interaction.
Other ongoing work in the De Strooper lab focuses on how transplanted human microglia respond to amyloidosis. In Lisbon, collaborator Renzo Mancuso of the University of Antwerp described results from these studies, which he conducted with De Strooper lab postdoc Anna Martinez-Muriana and graduate student Nicola Fattorelli, who now works as a postdoc in Mancuso’s lab. In Lisbon, Mancuso described their newest findings, which were published in Nature Neuroscience on March 27 (Mancuso et al., 2024). Alzforum previously covered the bulk of the findings as reported on bioRxiv (Oct 2022 news). Essentially, Mancuso reported that upon exposure to amyloidosis in APP-NL-GF mice, transplanted human microglia gradually ditch their homeostatic signature in favor of a number of transcriptional states. These included two related cytokine response microglia (CRM) states, which expressed a bevy of inflammatory chemokine and cytokine genes; a disease-associated microglia (DAM) state; and an antigen-presenting profile, which involved the upregulation of HLA genes. Mancuso reported that transitions into these states proceeded on two separate transcriptional trajectories, one leading to CRMs, and the other to DAM and then to HLA. Based on these and other findings, he speculated that perhaps CRM arise in response to Aβ oligomers, while they embark on the DAM/HLA trajectory in response to plaques. In support of this idea, the CRM state appears to arise earlier and hold steady as mice age, while the DAM/HLA state emerges later.
Collectively, these AD/PD presentations make it clear that microglia are a moving target, shifting their roles as disease progresses. Despite this, scientists are still developing therapeutics to coax the cells into neuroprotective states. Read Part 9 of this series (to come) to learn about microglia-targeted contenders presented in Lisbon.—Jessica Shugart
References
News Citations
- In Amyloid Cascade, Do Reactive Astrocytes Bridge Plaques and Tangles?
- Microglia Conflicted: To Help, or to Hinder, Tau’s March Across the Brain?
- Most Detailed Look Yet at Activation States of Human Microglia
- TREM2, Microglia Dampen Dangerous Liaisons Between Aβ and Tau
- Are Microglia Plaque Factories?
- Human Microglia Mount Multipronged Response to AD Pathology
Paper Citations
- DeJager RM, Lee AJ, Sigalov A, Taga M. An image segmentation pipeline optimized for human microglia uncovers sources of morphological diversity in Alzheimer's disease. 2024 Feb 07 10.1101/2024.02.01.577128 (version 1) bioRxiv.
- Zhao R, Hu W, Tsai J, Li W, Gan WB. Microglia limit the expansion of β-amyloid plaques in a mouse model of Alzheimer's disease. Mol Neurodegener. 2017 Jun 12;12(1):47. PubMed.
- Mancuso R, Fattorelli N, Martinez-Muriana A, Davis E, Wolfs L, Van Den Daele J, Geric I, Premereur J, Polanco P, Bijnens B, Preman P, Serneels L, Poovathingal S, Balusu S, Verfaillie C, Fiers M, De Strooper B. Xenografted human microglia display diverse transcriptomic states in response to Alzheimer's disease-related amyloid-β pathology. Nat Neurosci. 2024 May;27(5):886-900. Epub 2024 Mar 27 PubMed.
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.