In Hydrocephalus, Slow Drainage May Cause Dementia
Quick Links
Few scientists doubt that backed-up clearance of detritus from the brain correlates with accumulation of amyloid plaques and neurofibrillary tangles. But which comes first? Data presented at the 9th Kuopio Alzheimer Symposium suggests that, at least in some cases, the plumbing might be the problem. Per Kristian Eide, University of Oslo, Norway, reported that the cerebrospinal fluid does not drain properly in people who have normal-pressure hydrocephalus. Is there an AD connection, you may ask? In people with this brain condition, the incidence of dementia, and amyloid and tangle pathology, far exceeds that of the general population. Eide’s data support the idea that sluggish drainage of CSF may cause AD pathology.
- People with normal-pressure hydrocephalus have enlarged ventricles.
- Most also have a cognitive impairment.
- Molecules clear slowly from their brains, perhaps worsening Alzheimer's pathology.
A rare condition, idiopathic normal-pressure hydrocephalus typically manifests as a troika of symptoms, that is, a gradual loss of balance, bladder control, and cognition (Williams et al., 2016). It primarily affects older people, and about 6 percent of octogenarians are estimated to have the disorder (Jaraj et al., 2014; Iseki et al., 2022). Brain scans show that these people have enlarged ventricles filled with CSF.
On autopsy or biopsy, a small majority of people with NPH and dementia also have amyloid plaques and neurofibrillary tangles, the hallmarks of AD pathology (Cabral et al., 2011; Koivisto et al., 2016). In many cases, the tangles can be sparse, with little evidence of neurodegeneration, suggesting that there might be an opportunity to intervene early in their AD progression (Libard and Alafuzoff, 2019).
Indeed, some people who have a shunt surgically implanted in their brain to drain the CSF make remarkable recoveries and their cognition improves (Adams et al., 1965; McGirt et al., 2005; Liu et al., 2016). However, this is far from true for everyone. Researchers in Ville Leinonen’s lab at Kuopio University Hospital reported that four years after this procedure, 80 percent of people had cognitive decline and 46 percent had either Alzheimer's or vascular dementia (Koivisto et al., 2013).

Trace The Fluid. Serial MRI scans show that after injection into a healthy person's cerebrospinal fluid, the MRI contrast agent gadobutrol gradually spreads throughout his or her brain, depicting a normal glymphatic system. [Courtesy of Per Kristian Eide.]
What causes NPH? In some cases, called “noncommunicating NPH,” it can be a blockage that restricts CSF flow between the brain's ventricles. In “communicating NPH,” the obstruction occurs after CSF leaves the ventricles. In many cases the cause is unknown.
To study the flow of CSF in idiopathic NPH, Eide and colleagues tested the MRI contrast agent gadobutrol. This gadolinium compound is used in animals to image the glymphatic system, by which interstitial fluid moves through the brain and exchanges with the cerebrospinal fluid (Aug 2012 news; Mar 2013 news).
In Kuopio, Eide showed that when the tracer is injected into the spine of a healthy volunteer, it slowly spreads into the parenchyma of the brain (Ringstad et al., 2018). This distribution peaks up to a day later, Eide said (see image above). Because the resolution of MRI is only around 1 mm, it can't depict the exact path the tracer takes.
Even so, Eide showed that it flows beside arteries, without penetrating them. This is in keeping with the idea that fluid in the brain spreads by the paravascular glymph system, whereby CSF flows into the parenchyma along arteries, and drains out of it along veins (Aug 2012 news). In keeping with this, the strongest spread of gadobutrol into the parenchyma occurred around major arteries. Eide thinks that the pulsing of the arteries helps to gently push the fluid along.

Brain Drain? The MRI tracer gadobutrol makes its way from the CSF (light blue) to the parasagittal dura (yellow), which harbors lymph vessels that drain the brain (not shown). [Courtesy of Ringstad and Eide, 2020.]
Does this paravascular transport system change in iNPH? Geir Ringstad at Oslo University Hospital compared gadobutrol clearance among eight healthy controls and 15 people with iNPH (Ringstad et al., 2017). Ringstad found that, in iNPH, the tracer is slow to enter and exit the Sylvan fissure. This major sulcus separates the frontal and parietal lobes from the temporal lobe. It is a main point of entry for gadobutrol into the parenchyma from the CSF. What’s more, once the agent was in the parenchyma, it cleared more slowly than it did in healthy controls.
Following these findings, Eide and colleagues modeled how gadobutrol drains from the brain into the blood. First, some background. Scientists know that the meninges and dural membranes of mouse and human brains contain a set of lymph vessels that likely sieve interstitial fluid pushed through the brain by the glymph system (Aspelund et al., 2015; Louveau et al., 2015; Oct 2017 news). Eide and colleagues had found that gadobutrol squeezes from the interstitial fluid into the parasagittal dura at the top of a person's brain, which houses some of these lymph vessels (Ringstad and Eide, 2020). Eide believes that the parasagittal dura serves as a bridge between the CSF and the lymph, and ultimately, the blood.
Now for the blood modeling. Markus Herberg Hovd and colleagues in Eide’s lab injected gadobutrol into the CSF of 161 volunteers, then measured it in blood samples taken at intervals for up to 48 hours (Hovd et al., 2022). Of these people, 28 were healthy controls, 63 had iNPH, and 70 had another form of hydrocephalus or CSF disorder. At the Kuopio conference, Eide showed how Hovd used the blood data to model the pharmacokinetics of brain clearance, arriving at a formula that closely matched the empirical data.
He then plugged blood sample data for each volunteer into the formula to determine clearance pharmacodynamics of each volunteer (see graphs below), including how long it took half of the tracer to clear the CSF, time to maximum concentration in the blood (Tmax), the peak concentration in blood (Cmax), and the lag time before the tracer appeared in the blood (Tlag).
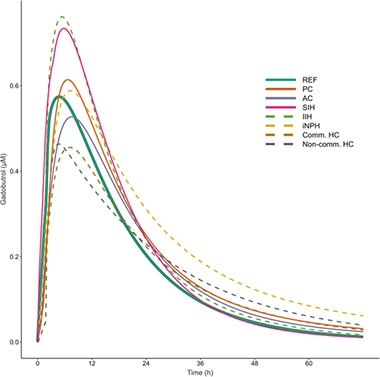
Clearance Problems. Modeling CSF clearance by tracking the appearance of gadobutrol in the blood suggests that people with iNPH (yellow dashes) take longer to clear solutes from the brain than do healthy controls (solid green). Pharmacodynamics also changed in people with other forms of NPH (PC, pineal cyst; AC, arachnoid cyst; SIH, spontaneous intracranial hypotension; IIH, idiopathic intracranial hypertension; Comm HC, communication hydrocephalus; Non-comm HC, non-communicating hydrocephalus). [Courtesy of Hovd et al., 2022.]
The first thing Hovd noticed was that the model indicated that the volunteers, even the controls, were markedly different from one another on all those parameters. “This considerable variability really surprised us,” said Eide. Still, differences emerged between the groups. For example, in 11 people who had communicating hydrocephalus, the Cmax was about 30 percent lower than in controls. In 13 people who had a pineal cyst, Tlag was more than twice as long. That said, people with iNPH had the most dramatic changes. Both their Tlag and Tmax were longer than in the reference group, meaning the tracer hung around longer in their brains. Ultimately, gadobutrol reached higher concentrations in the blood of people with iNPH, but Eide thinks this happened because their kidneys were affected, too, and cleared the tracer more slowly.
It remains to be seen whether this abnormal CSF drainage in iNPH contributes to AD pathology, as has been shown in animal models. Next, Eide plans to measure gadobutrol clearance in people with mild cognitive impairment.—Tom Fagan.
References
News Citations
- Brain Drain—“Glymphatic” Pathway Clears Aβ, Requires Water Channel
- Spinal Fluid Flush: Visualizing the Brain Drain With MRI
- Lymphatic Vessels Found in Human Brain
Paper Citations
- Williams MA, Malm J. Diagnosis and Treatment of Idiopathic Normal Pressure Hydrocephalus. Continuum (Minneap Minn). 2016 Apr;22(2 Dementia):579-99. PubMed.
- Jaraj D, Rabiei K, Marlow T, Jensen C, Skoog I, Wikkelsø C. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology. 2014 Apr 22;82(16):1449-54. Epub 2014 Mar 28 PubMed.
- Iseki C, Takahashi Y, Adachi M, Igari R, Sato H, Koyama S, Ishizawa K, Ohta Y, Kato T. Prevalence and development of idiopathic normal pressure hydrocephalus: A 16-year longitudinal study in Japan. Acta Neurol Scand. 2022 Nov;146(5):680-689. Epub 2022 Sep 16 PubMed.
- Cabral D, Beach TG, Vedders L, Sue LI, Jacobson S, Myers K, Sabbagh MN. Frequency of Alzheimer's disease pathology at autopsy in patients with clinical normal pressure hydrocephalus. Alzheimers Dement. 2011 Sep;7(5):509-13. PubMed.
- Koivisto AM, Kurki MI, Alafuzoff I, Sutela A, Rummukainen J, Savolainen S, Vanninen R, Jääskeläinen JE, Soininen H, Leinonen V. High Risk of Dementia in Ventricular Enlargement with Normal Pressure Hydrocephalus Related Symptoms1. J Alzheimers Dis. 2016 Mar 22;52(2):497-507. PubMed.
- Libard S, Alafuzoff I. Alzheimer's disease neuropathological change and loss of matrix/neuropil in patients with idiopathic Normal Pressure Hydrocephalus, a model of Alzheimer's disease. Acta Neuropathol Commun. 2019 May 29;7(1):98. PubMed.
- ADAMS RD, FISHER CM, HAKIM S, OJEMANN RG, SWEET WH. SYMPTOMATIC OCCULT HYDROCEPHALUS WITH "NORMAL" CEREBROSPINAL-FLUID PRESSURE.A TREATABLE SYNDROME. N Engl J Med. 1965 Jul 15;273:117-26. PubMed.
- McGirt MJ, Woodworth G, Coon AL, Thomas G, Williams MA, Rigamonti D. Diagnosis, treatment, and analysis of long-term outcomes in idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005 Oct;57(4):699-705; discussion 699-705. PubMed.
- Liu A, Sankey EW, Jusué-Torres I, Patel MA, Elder BD, Goodwin CR, Hoffberger J, Lu J, Rigamonti D. Clinical outcomes after ventriculoatrial shunting for idiopathic normal pressure hydrocephalus. Clin Neurol Neurosurg. 2016 Apr;143:34-8. Epub 2016 Feb 9 PubMed.
- Koivisto AM, Alafuzoff I, Savolainen S, Sutela A, Rummukainen J, Kurki M, Jääskeläinen JE, Soininen H, Rinne J, Leinonen V, Kuopio NPH Registry (www.uef.finph). Poor cognitive outcome in shunt-responsive idiopathic normal pressure hydrocephalus. Neurosurgery. 2013 Jan;72(1):1-8;discussion 8. PubMed.
- Ringstad G, Valnes LM, Dale AM, Pripp AH, Vatnehol SS, Emblem KE, Mardal KA, Eide PK. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight. 2018 Jul 12;3(13) PubMed.
- Ringstad G, Vatnehol SA, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. 2017 Oct 1;140(10):2691-2705. PubMed.
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015 Jun 29;212(7):991-9. Epub 2015 Jun 15 PubMed.
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 Jul 16;523(7560):337-41. Epub 2015 Jun 1 PubMed.
- Ringstad G, Eide PK. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat Commun. 2020 Jan 17;11(1):354. PubMed.
- Hovd MH, Mariussen E, Uggerud H, Lashkarivand A, Christensen H, Ringstad G, Eide PK. Population pharmacokinetic modeling of CSF to blood clearance: prospective tracer study of 161 patients under work-up for CSF disorders. Fluids Barriers CNS. 2022 Jul 1;19(1):55. PubMed.
Further Reading
Papers
- Thomas G, McGirt MJ, Woodworth G, Heidler J, Rigamonti D, Hillis AE, Williams MA. Baseline neuropsychological profile and cognitive response to cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord. 2005;20(2-3):163-8. Epub 2005 Jul 15 PubMed.
- Eide PK, Pripp AH, Berge B, Hrubos-Strøm H, Ringstad G, Valnes LM. Altered glymphatic enhancement of cerebrospinal fluid tracer in individuals with chronic poor sleep quality. J Cereb Blood Flow Metab. 2022 Sep;42(9):1676-1692. Epub 2022 Mar 29 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.